Cyclo.Plas 2: A Dual Focus Development as Alternative Materials to Plastic by Upcycling Fish Scale Waste Components
Jacqueline Prawira1
1Mountain House High School
Youth STEM Matters, no. 1 (2021); https://doi.org/10.51892/ysm.1.202104J. Prawira, "Cyclo.Plas 2: A Dual Focus Development as Alternative Materials to Plastic by Upcycling Fish Scale Waste Components," Youth STEM Matters, no. 1, 2021.
If you cite this article or use it in any academic activities please let us know by e-mailing us at editor@youthstem2030.org.
Abstract
Introduction
80 million tons of fish were caught in 2016. After the fish flesh was harvested, 20-80% of the fish, including fish bones, fins and scales, was discarded as processing waste [1]. The volume of fish waste produced can cause handling difficulties, leading to landfills filling up or improper disposal in the ocean. That can drastically lower available dissolved oxygen for nearby organisms, cause local eutrophication (episodes of excess nutrients causing unhealthy growth of plants and animal deaths), bury seafloor communities, and introduce diseases or non-native species into other areas [2].
Fish scales, one of the inedible byproducts of fish, are dermal bones. Cycloid and ctenoid fish scales, which are found in the bony fish caught by fisheries, are generally composed of: an outermost layer of bone; an organic framework filled with calcium salts (primarily HAp); and a bottom layer of protein (mostly fibrous collagen) [3]. Collagen from fish scales is mostly the highly abundant Type I collagen, which forms the foundations of human skin and bones and has a tensile strength stronger than steel [4]. When heated beyond their shrinkage temperature, these fibrils irreversibly shrink due to a disassembly of fibers and a collapse of their triple-helix structure [5]. Hydroxyapatite has a high flexural strength of up to 120 MPa, comparable to PLA. It is composed of calcium, phosphate, and hydroxide ions, and forms a hexagonal crystal structure [6, 7]. As an ionic solid, it demonstrates brittleness due to the attraction and repulsion of ionic charges resisting crystal deformation [7]. Hydroxyapatite undergoes biomineralisation and bonds with the organic framework to form calcified tissues and provide density and durability [8]. It also dissociates in water and demonstrates increasing solubility in acidic solutions due to neutralisation reactions with the two anions, meaning that it can degrade naturally in the environment [9, 10].
Simultaneously, the proliferation of plastics in society has resulted in widespread dependency on plastic and its accumulation in the environment. The demand for convenience has made plastic products integral to most people’s daily lives, with 8.3 billion tons of plastic produced per year [11]. As a result, 300 million tons of plastic are discarded each year, with around 60% of that plastic ending up in a landfill or in the environment. The problem is that, given the continuous consumption of plastics, this could result in the oceans containing more plastic than fish by 2050. Therefore, research has focused on improving the biodegradability of these plastics to reduce landfill waste, thereby also preventing ocean pollution [11].
Polylactic acid filament, one of the most used materials in 3D-printing, is sourced from renewable biomass and can be composted or recycled. As a result, its prevalence as an alternative to petroleum-based plastics is growing. However, PLA can only properly compost in scarce industrial composting facilities [12]. Without the high temperature and large microbe population of the industrial facilities, PLA’s slow degradation rate means that it essentially behaves just like plastics in the environment. Additionally, the recycling process often creates weakened, inconsistent and brittle filaments, with tensile strength reduced from an average of 40 MPa to 35 MPa, thus increasing chances of breakage and warping and making it much harder to work with [13]. Ultimately, due to the lack of options in composting or recycling PLA, it ends up in a landfill or polluting the environment, much like regular plastics.
The lack of degradability of plastic and the lack of compostability of common bioplastics (such as PLA) is the source of the plastic problem. Additionally, fish scales are commonly considered waste and have no industrial value. This means that valuable sources of collagen and hydroxyapatite are often thrown away. Unlike plastics, fish scale waste has the benefit of being able to degrade back into its environment due to its composition. The collagenous matrix forms a translucent, durable material as the inner fibrous layer of the scale, while calcium salts (such as HAp) bond with the organic matrix to harden the tissue and provide structure through biomineralisation. However, this matrix is susceptible to degradation, resulting in a loss of structural integrity. Crosslinking these collagen fibres with more robust materials will therefore improve their stability under varied environmental conditions. One such potential material is chitosan, a water-insoluble polysaccharide, which cross-links with collagen in a process called sclerotisation, resulting in the hardening of the composite polymer [4, 5]. This demonstrates the potential to manipulate collagenous matrix as a thin film which serves as an environmentally friendlier alternative to current thin film plastics.
The calcium salts in fish scales provide structure and rigidity, whilst 3D-printed PLA lacks compostability and recycling prospects [3, 6]. This is because repeated melting and cooling cycles break the polymer chains in PLA, resulting in the degradation of its quality [13]. Hence, valorisation of 3D-printed PLA waste using fish scale calcium salt could restore the strength of PLA while also enhancing its compostability/degradability in the environment, because the calcium salts in fish scales undergo biomineralisation with an organic framework to provide additional strength. Applying biomimicry of the biomineralisation concept, the presence of calcium salts in a PLA composite could bond with the PLA chains to enhance strength. During degradation, the incorporation of calcium salts could accelerate the rate of hydrolysis by creating a higher surface area to volume ratio. This enhancement in strength and compostability indicates the potential of the composite material, dubbed CP2-composite, in replacing plastics.
Previous research successfully upcycled fish scale waste by isolating the collagenous matrix to form a thin, 74% translucent, plastic-like material named Cyclo.Plas (unpublished data). Using the intact collagenous matrix for structure, tensile strengths reached up to 53.77 MPa, comparable to PLA and LDPE. With a 93% processing yield, the cost of Cyclo.Plas at $3.03/kg is competitive. Cyclo.Plas had higher shrinkage performance from 130-150°C, whereas LDPE melted and deformed. Cyclo.Plas degraded within 5 weeks in both soil and hydroponic environments, with a 100% seedling survival rate. Plant growth varied between 96%-108%, with no signs of phytotoxicity, including chlorosis, necrosis and wilting. The key to Cyclo.Plas’s degradation is its composition being almost 100% protein. Critical parameters were established for successful formation for real-world applications and prototypes were successfully created, with the potential to replace thin film packaging and the ability to easily hold approximately 7kg.
Whilst Cyclo.Plas demonstrated high tensile performance and rapid, non-toxic degradation, the water resistance and thermal stability reduced the number of potential applications. At the same time, improving the degradation rate of PLA is crucial to reducing the reliance on compositing facilities. Thus, the aim of this study is to tap fish scale waste components with a dual focus as a composite and a thin film alternative to plastic. The concept of biomineralisation was used to valorise 3D-printed PLA waste with fish scale inspired materials, forming a reinforced composite with enhanced compostability. The concept of sclerotisation was used to enhance the properties of a thin film from the intact collagenous matrix of fish scale waste, successfully boosting the thermal and water resistance. The strength and degradability of fish scale waste components are the keys to Cyclo.Plas 2’s corresponding enhanced properties and therefore its viability as an alternative to current plastic materials.
Methods
Synthesis of PLA/HAp Composite
3D-printed PLA waste was combined with HAp, which provides structure, in ratios 90/10, 80/20, 70/30, and 60/40, to form the material: CP2-composite. Stearic acid (SA) acted as a compatibiliser/emulsifier in various amounts (10%, 20%, 30% and 40%) to evaluate the effects on the resulting composites’ strength and compostability (Table S1). Unmodified 3D printed PLA waste was used as a performance control in all Focus 1 tests.
Flexural Strength Testing
Flexural strength testing measured the strength of the various CP2-composite ratios and quantified the enhancement to determine the degree of success the valorisation process had in remedying PLA’s loss in strength with repeated melting/cooling.
Microscopy
Microscopic Surface Analysis compared the effects of physical stress and degradation on the composite surface using a digital microscope. Scanning Electron Microscope (SEM) analysis examined the degree of homogeneity in the composite and the effects of physical stresses and degradation on its structure. All samples were imaged in a TM4000 SEM with magnifications 10x-250,000x inside a vacuum-sealed chamber.
Degradation Testing
Hydrolysis at 70°C measured the impact of HAp and SA on the rate of degradation at elevated temperatures over one week. Simulated aquatic degradation measured the rate of decomposition by mass loss and turbidity of the testing environment in pH levels 5-9, with full-spectrum lamps to simulate photodegradation in sunlight and a spectrophotometer to quantify turbidity over four weeks. The composting test measured the suitability of the CP2-composite in home composting (using a home compost bin) and compared it to the standard of compostability, American Society for Testing and Materials (ASTM) D6400 for PLA in industrial composting facilities over 12 weeks.
Synthesis of Collagen/Chitosan (Co/Ch) as Thin Film
Re-processed Cyclo.Plas samples were used to source collagenous proteins, with emphasis on recovering the intact collagenous matrix. Re-hydrolysis at 55℃ for one hour deconstructed the thin film matrix through partial hydrolysis to combine it with chitosan and enhance its properties. The collagenous mixture (10% w/v sample in distilled water) was then recombined with a chitosan solution (5% w/v of chitosan in 1% acetic acid) using the concept of sclerotisation to enhance water resistance and thermal stability in ratios 90/10, 70/30, and 50/50. Cooling/drying set the collagenous proteins and formed cross-linkages with chitosan, using a self-designed/homemade cooling chamber, circulating RT/20°C air for 72 hours. Thermal dehydration strengthened the thin film matrix by removing water molecules and by forming thermal bridges at 40℃, 55℃, and 70℃ for eight hours each. Re-hydrolysed Cyclo.Plas without chitosan was used as a performance control in all Focus 2 tests to compare with the new thin film material, CP2-thin film.
Physical Testing
Tensile strength testing measured the physical properties and compared to regular thin plastics used in similar applications based on ASTM D882, the tensile strength standard for thin-film plastics less than 1 mm in thickness [14]. Linear dimensional shrinkage characterised the stability at elevated temperatures 80-150℃, and it was compared to regular thin plastic samples from grocery store plastic bags using ASTM 1204, which measures dimensional change in the x- and y- directions in response to heat [15]. Transparency testing characterised the changes in light transmittance at room and elevated temperatures 80-150℃ using ASTM 1746, which measures changes in transparency due to heat [16].
Environmental Testing
Phytotoxicity testing measured plant growth while evaluating phytotoxicity, and potential effects on the environment based on OECD 208, which measures phytotoxicity in soil, for four weeks. Biodegradation testing measured the rate of degradation in soil in eight weeks [17]. Water solubility testing measured water solubility to determine degradability and potential impact on aquatic environments for one week.
Results
Testing the Physical Properties of PLA/HAp/SA Composites
The relationships between PLA, hydroxyapatite (HAp), and stearic acid (SA) influences the composite formation. PLA serves as the matrix material, while HAp provides structure, like the biomineralisation concept. SA functions as a compatibiliser to combine PLA and HAp despite the polarity differences. Composites consisting of different ratios of PLA, HAp, and SA (Table S1) were constructed to examine their physical properties.
Increased composite PLA content improves flexural strength
Having constructed composites consisting of different ratios of PLA, HAp and SA, their strength, and the success the valorisation process had in remedying PLA’s loss in strength was determined by repeated melting and cooling. Overall, the flexural strength of the PLA/HAp composites was higher than the control samples (Fig. 1). Even compared to the Makerbot PLA filament datasheet with flexural strengths between 61-95 MPa, the PLA/HAp composites had higher performance. On average, the 90/10 PLA/HAp ratio displayed high flexural strength, whereas ratios 80/20, 70/30, and 60/40 all showed similar flexural strength, suggesting that HAp improves the structural integrity of the material.
Figure 1 - Average flexural strength of PLA/HAp Composite Ratios. Composites consisting of different ratios of PLA to HAp were constructed: their flexural strengths were measured by repeated melting and cooling. This data was sourced from 3-point flexural strength tests, with each data point being averaged from three trials consisting of two tested samples each.
A higher composite HAp content increases the brittleness of the material
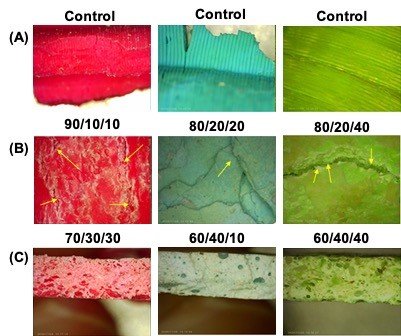
After subjecting the composites to physical stress and the degradation process, microscopic analysis was used to visualise and compare the effects on the surface of the material. Higher PLA ratios demonstrated a smoother texture and consistency. Conversely, higher HAp ratios demonstrated a grainier texture: there were some small clumps of HAp throughout, with more clumping at higher HAp ratios. Higher amounts of SA increased the number of voids inside the composites (Fig. 2). After flexural strength testing samples demonstrated increasingly brittle failure as the ratio of HAp increased. This was demonstrated by the reduction in miniature “necking”-like features along the break surface (Fig. 2, arrows). This indicates that the ratios of the components had an apparent impact on the blending and formation of the composite.
Figure 2 - Average flexural strength of PLA/HAp composite ratios. Composites consisting of different ratios of PLA to HAp were constructed: their flexural strengths were measured by repeated melting and cooling. (A) Control group of 3D-printed PLA waste. (B) These samples exhibited more ductile formation and had more widely distributed areas of failure. (C) These samples exhibited a single primary fracture. This data was sourced from 3-point flexural strength tests, with each data point being averaged from three trials consisting of two tested samples each.
The presence of HAp accelerates the rate of composite degradation
Since HAp has been shown to alter the structure of the composite, SEM analysis was conducted to examine the degree of homogeneity in the composite and establish the effects of physical stresses and degradation on its structure. The samples’ cross-sections reveal a porous inner structure (Fig. 3). Additionally, in a composting soil environment, it appears that soil microbes formed fibrous growths and created holes on the surface of the samples, leading to even faster breakdowns (Fig. 3). Higher HAp ratios showed greater numbers of pores, which may contribute to the increased degradation rate. In contrast, the control samples showed minimal degradation. This indicates that the presence of hydroxyapatite can accelerate the rate of degradation, as hydroxyapatite can dissociate into its constituent ions.
Figure 3 - SEM analysis images. Scanning electron micrographs were taken at magnifications x100, x200 and x300 to examine the structure of the composite following hydrolysis at 70°C or a home compost. Blue circles indicate areas where pores are visible in the structure. Red arrows indicate holes created by the growth of microbes while red circles indicate clusters of the filamentous microbe growths.
Composite Degradation in Varying Conditions
Composite degradation rates are increased by higher ratios of both HAp and SA
Having established the effects of HAp on the structure and physical properties of the composites, the impacts of both HAp and SA on the rate of degradation were investigated following hydrolysis of the material at 70°C over a one-week period. It was found that higher HAp ratios accelerated the degradation rate on average whereas higher ratios of PLA slowed down the degradation rate. (Fig. 4A). Sample degradation varied between 24 to 36%. Higher amounts of SA also increased the rate of degradation, but with a lesser impact (Fig. 4B). This indicates that the ratio of PLA to HAp influenced the rate of degradation.
Figure 4 - The effects of HAp and stearic acid on hydrolysis at 70°C. Mass reduction in the presence of varying ratios of HAp (A) and stearic acid (B) was used to determine degradation rates over a one-week period. This data was derived after weighing the dry mass of samples experiencing one week 70℃ hydrolysis in distilled water. Each data point is averaged from three trials.
Composite degradation rates are increased in acidic conditions
Given that the structural makeup of the composite can influence the degradation rate, environmental factors, such as pH, were investigated to determine whether they also had an effect. To measure the rate of degradation, simulated aquatic degradation was conducted at pH levels 5-9 to measure mass loss and turbidity of the degradation environment over four weeks. Overall, more acidic environments showed higher levels of degradation (Fig. 5). This indicates that the pH of the solution and the HAp ratios had the greatest influence on the degradation rates. Higher HAp ratios also resulted in a higher rate of degradation overall (Fig. 5A), and increased quantities of SA also increases the rate of degradation (Fig. 5B). Furthermore, to assess the changes in transparency, full-spectrum lamps were used to simulate photodegradation in sunlight. The pH of the solution influenced the overall transparency over a period of four weeks. The lower the pH level, the higher the transparency, while increased pH lowers the transparency (Fig. 5C and D). This data suggests that acidic environments minimise the interaction of HAp and SA with other water constituents.
Figure 5 - Effect of HAp and SA on the degradation rates of PLA/HAp composites at pH levels 5-9. Composites consisting of different amounts of Hap (A, C), and SA (B, D) were subjected to simulated aquatic degradation at pH levels 5-9 over a period of four weeks. White bar = 90/10 PLA/Hap or 10% SA, light grey bar = 80/20 PLA/Hap or 20% SA, dark grey bar = 70/30 PLA/Hap or 30% SA and black bar = 60/40 PLA/Hap or 40% SA. Mass reduction (A and B) was measured from dried samples after four weeks of submersion in solutions at pH levels 5-9 under full-spectrum lights (data are averaged from three trials). Transparency changes (C and D) were derived from transparency readings of aliquoted solutions after four weeks of testing (data are averaged from four trials).
Biodegradability increases with the PLA/HAp composites in home composting systems than PLA alone
As the composites were found to have altered degradability in aquatic environments, research was conducted to examine their degradation in home composting systems. To measure the suitability of the PLA/HAp composites in home composting and compare this to the standard of compostability for PLA in industrial composting facilities, a home compost bin was used to facilitate the composting test. CP2-composite successfully degraded in home composting environments over 12 weeks by approximately 7% (Fig. 6). Compared to control, CP2-composite degraded by over three-fold. The changes in the HAp and SA ratios do not seem to have a large effect on the rate of decomposition during the first 12 weeks. This data suggests that HAp increases the biodegradability of the composite, thus making it more environmentally friendly compared to standard PLA.
Figure 6 - Mass reduction in home composting after 12 weeks. The mass reduction data was measured from samples after 12 weeks in home composting conditions. Each data point is averaged from three trials.
Cyclo.Plas 2 Property Testing
CP2-thin film was successfully formed in all three ratios of collagen/chitosan: 90/10, 70/30, 50/50, producing translucent, flexible, thin sheets with a slight pale yellow colour. Re-processed Cyclo.Plas samples were used to source the collagenous proteins required, with particular emphasis on recovering the intact collagenous matrix.
CP1 and CP2 have minor differences in tensile strength
To measure physical properties and compare them to regular thin plastics used in similar applications based on ASTM D882, tensile strength testing was conducted. CP2-thin film with 90/10 ratio had the highest overall average tensile strength, followed by 70/30 ratio and 50/50 ratio (Fig. 7). Compared to CP1, the tensile strength of CP2-thin film in all ratios is comparable, with up to 1.2% variation. Compared to re-hydrolysed CP1 control (where the collagenous matrix is broken down) with lowered tensile strength, the tensile strength of CP2-thin film appears to be slightly improved. Statistical analysis and more trials would be needed to confirm the magnitude of this enhancement.
Figure 7 - Ratios of Co/Ch and their effect on tensile strength. Each of the four ratios tested underwent five trials, which were averaged for each data point on the graph. The “CP1 Original” data point is derived from prior data obtained (unpublished).
CP2 is more stable at elevated temperatures compared to CP1
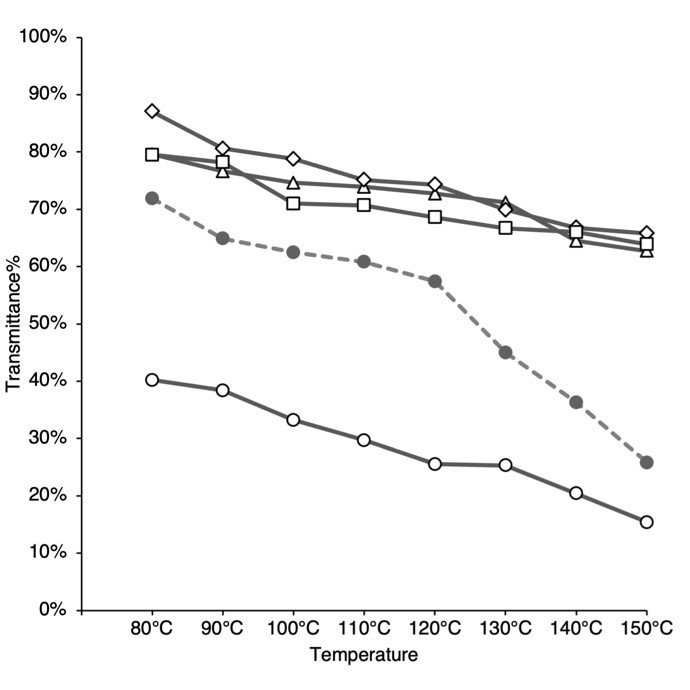
Having established that CP2 has no major difference in tensile strength to CP1, its stability at elevated temperatures (80-150℃) was compared to regular thin plastic samples from grocery stores’ plastic bags using ASTM 1204. CP2-thin film, in all ratios, shows the lowest maximum shrinkage of 25% - 31% compared to 32%-35% for CP1 (control), 50% - 54% for thin LDPE and 35%-44% for PLA compost bag at 140°-150°C (Fig. 8). The shrinkage was higher in the 80-90°C range but was minimal between 100°-150°C. The higher chitosan content in CP2-thin film ratios generally reduces the shrinkage at 100°C and above. Hydrolysed CP1 has the highest shrinkage compared to the CP1 and CP2-thin film in all ratios. This suggests that the presence of chitosan enhances thermal stability. On the other hand, the re-hydrolysis process appears to have reduced thermal stability.
Figure 8 - Linear dimensional shrinkage as a function of temperature ASTM D1204. CP1 (--●--), CP1 Hydrolysed (––o––), CP2 90/10 (––△––), CP2 70/30 (––◇––), CP2 50/50 (–––⃞–), Thin LDPE (··x··), PLA trash bag (··+··). After exposure to various temperatures (80-150℃) for 1 hour, the dimensional changes in the x and y-directions were measured and averaged. Each data point is averaged from 6 trials.
CP2 has a higher transparency up to 150°C compared to CP1
To characterise the changes in light transmittance at room and elevated temperatures between 80-150℃ using ASTM 1746, transparency testing was facilitated. CP2-thin film is translucent with 86% transmittance to visible light (550 nm) for samples with 0.53mm in thickness (Fig. 9). Compared to CP1, CP2-thin film maintains higher transparency across all temperatures. Elevated temperatures reduce the transparency of CP2-thin film and change the colour from pale yellow to light brown. The transparency is reduced almost linearly across all CP2-thin film ratios, with an average reduction of 2.4% in transparency with every 10% increase in temperature. This further suggests that the thermal resistance of the chitosan is increasing the thermal stability.
Figure 9 - Sheet transparency as function of temperature ASTM D1204 - 550nm light source. CP1 (--●--), CP1 Hydrolysed (––o––), CP2 90/10 (––△––), CP2 70/30 (––◇––), CP2 50/50 (–––⃞–), Thin LDPE (··x··), PLA trash bag (··+··). Samples from Linear Dimensional Shrinkage testing underwent transparency testing, with 4 trials being averaged for each graphed data point.
CP2 presence in soil has no negative impact on plant growth
Often degradation of chemicals can result in the release of toxic by-products which can negatively subsequently affect crop yields. Therefore, to assess for any potential negative environmental impacts of CP2, phytotoxicity testing was conducted for four weeks to measure plant growth and phytotoxicity based on OECD 208. All plants showed no signs of phytotoxicity with a 100% survival rate. Each seed type grows at different rates, the fastest being wheatgrass, followed by lentil and mung bean (Fig. 10). Compared to control, plant growth was 3%-7% higher overall. This data suggests that the presence of CP2 in the soil is not harmful to plant growth but may instead provide beneficial factors that enhance growth.
Figure 10 - Plant growth comparison in soil with various CP2-thin film amounts. White = control, light grey = 10g, dark grey = 20g and black = 30g. Phytotoxicity testing was conducted for four weeks to measure plant growth compared to the control as a percentage. Data was collected by measuring plant height. Each graphed data point is averaged from 16 plants. For each trial, three measurements were averaged.
CP2 successfully biodegrades in soil
Having established that CP2 is not harmful to plants in the environment, the ability of the material to degrade in soil was determined by measuring the rate of degradation in soil for eight weeks. CP2-thin film was proportionally (10g, 20g, and 30g) mixed with 1kg of organic soil, which was divided up into containers. All CP2-thin film successfully biodegraded in the soil within eight weeks. In soil, the CP2 samples broke down starting week two (Fig. 11). The remaining scale matrices were softened, and biodegraded further after six weeks, with little visible traces left by the end of the eighth week. Compared to CP1 (collagen only), CP2 with chitosan content improves water resistance, yet is still biodegradable. These data suggest that it would be appropriate and safe to dispose of CP2 in the ground when it is being disposed of.
Since CP2 was shown to biodegrade in soil, its ability to degrade in aquatic environments was investigated along with any potential impacts on these environments. To determine degradability and potential impacts on aquatic environments, water solubility was measured for one week. CP2-thin film was partially dissolved in 250ml distilled water at room temperature between 17°-19°C after one week (Fig. 12). Increasing ratios of chitosan in CP2 reduced swelling and increased the mass retained compared to the control, CP1. The greater the total dissolved solids (TDS), the lower the degree of water resistance, due to the increased mass dissolved in the water. Interestingly, CP2 samples demonstrated curling and increased rigidity while the control samples remained flat once dried. These findings suggest that the recombination with chitosan has enhanced the water resistance of the samples.
Figure 11 - Observation of biodegradation in soil as a % of solid matter remaining. Varying masses of CP2 were added to 1kg of soil and the ability of the material to biodegrade was followed over an eight-week period. Each data point was averaged from 64 samples.
Figure 12 - Solubility/resistance in water after one week. Black bars = TDS, white bars = average % weight. Higher ratios of chitosan in CP2 increases water resistance as chitosan is water insoluble, as supported by the reduced mass loss and lower TDS readings. According to EPA standards, the TDS limit is 500 ppm. The mass loss was calculated from the starting dry mass of the samples and the dry mass after one-week-submergence in water. The TDS was measured from the water sample following the one week test using a TDS meter. Each graphed data point is averaged from three trials.
Discussion
Valorisation of 3D-Printed PLA Waste as Composite Material
To generate an optimised polymer, PLA and HAp components were selected, constituting the matrix and reinforcement materials respectively. A PLA to HAp ratio of 60/40 appears to be the optimal compromise between maximising HAp content and maintaining sufficient PLA to act as a medium for incorporating other components in the matrix. Furthermore, SA can compatibilise the nonpolar PLA and polar HAp through its amphipathic structure, having both a polar head and a nonpolar tail region [18]. It appears that a moderate amount of SA of 20-30% is sufficient to homogenise all PLA to HAp ratios. As HAp is a calcium salt, its ionic structure is key to the increased brittle deformation and reinforced strength of the upcycled PLA 3D printed waste; observations of the repeated melting and cooling cycles from 3D-printing demonstrated that the flexural strength reached a maximum of roughly 170 MPa. Although HAp has a flexural strength of 100-120 MPa (Fig. 1), increased HAp ratios in the composite lead to slight reductions in overall strength, possibly due to the higher ion presence interfering with polar interactions between the hydroxyl and carbonyl groups in crosslinking PLA chains. The absence of correct cross-linking would then result in a non-uniform matrix, generating the larger pore structures seen in the SEM analysis (Fig. 3). These pores would then increase the surface area of the composite exposed to the environment, therefore accelerating the PLA hydrolysis rate and impacting the overall structural integrity of the composite [19].
Degradation Rates
A key disadvantage to current bioplastics is their limited compostability, as many are unable to degrade outside industrial facilities. Thus, degradation experiments were conducted to assess the properties of the different composites, to identify the optimal compromise between structural integrity and compostability in the home. Towards this, water- and pH-mediated degradation rates were determined. The enhanced mass loss of CP2-composite as compared to controls is primarily influenced by a lower PLA/HAp ratio, most likely due to the increased hydrolysis rate of HAp compared to the degradation rate of PLA (Fig. 4). Additionally, the dissolved HAp left behind voids as observed under SEM (Fig. 3), creating more surface area for the PLA to be hydrolysed [20].
Similarly, an increased ratio of HAp leads to higher susceptibility of pH-induced degradation. The enhanced mass loss in acidic environments mainly results from the increased solubility of HAp in acidic solutions (Fig. 5). Higher concentrations of H+ ions in the local environment induce a shift in equilibrium towards the dissociated form of the HAp complex, leading to the release of the two anions present in HAp and therefore affecting the degradation of the composite. In acidic solutions, HAp is less likely to form insoluble precipitates, maintaining transparency [21]. However, in alkaline solutions, dissolved SA undergoes a process like saponification, essentially turning it to soap. When combined with the calcium ions from dissolved HAp, an insoluble precipitate forms, clouding the water [22, 23]. This suggests several applications; for water bodies affected by acidic pollution or acid rain, CP2 has potential to buffer the pH and maintain environmental balance. In a more alkaline environment, a greater concentration of OH ions would allow the hydrolysis of PLA to happen at a faster rate, degrading the PLA faster.
The level of degradation by mass (Fig. 6) observed in the home composting experiments may have been underestimated due to the additional mass of residual soil and microbe growths on the samples and the cold weather, which may lower the composting bin temperature. However, the samples were visibly degraded and more fragile compared to the original state, most likely due to the ionic structure of HAp allowing for replenishment of soil nutrients [24]. This shows the potential of CP2-composite to promote home composting as a waste management solution and its minimal potential impact when degrading in the environment.
Photodegradation from the full spectrum lamps was a constant across all samples and pH levels. However, photodegradation may have accelerated degradation for all samples, as photons are absorbed by pigments, accelerating the rate of oxidation and hydrolysis. The precipitation of HAp on the samples during degradation in various pH may have artificially deflated the rate of degradation; further experiments are necessary to assess these factors.
These findings suggest that a composite polymer made from different ratios of PLA, HAp, and SA can be used to alter the properties of the composite and cover a broader range of applications, whilst also proving the viability of the valorisation process on 3D-printed PLA waste. Single-use items requiring durable, rigid forms, such as non-medical PPE face shield frames, disposable utensils, and novelty toys, would be viable applications for this composite. CP2-composite has demonstrated both high flexural strength and enhanced compostability based on testing and prototype quality.
Enhancing the Properties of Cyclo.Plas as a Thin Film Material
Thermal re-hydrolysis of the collagenous matrix can cause the loss of structural integrity of the triple helix structure; the physicochemical properties of collagenous films will be affected through the presence of crosslinking polymers [4, 5, 14-25]. This is caused by the unfolding of collagen fibrils which promotes interconnections that create a film matrix [2, 5]. To broaden the scope of application of CP2, a novel thin film was synthesised via sclerotisation of chitosan and collagen polymers, leading to increased tensile strength when compared to controls [26]. With comparable tensile strength to LDPE (Fig. 7), this indicates that the prototypes already have the strength of current thin-film plastic products.
Chitosan's higher thermal resistance compared to collagen contributes to the robustness of the samples under increasing temperatures (Fig. 8); in contrast, LDPE plastics melt at around 130°C, resulting in sudden linear dimensional shrinkage and deformation [25, 29, 30, 31]. Interestingly, although material transparency was also reduced at elevated temperatures, transparency was retained to a higher degree in CP2 film when compared to CP1 (Fig. 9). This may be due to thermal resistance and the slight yellow colouration from chitosan, which increases transmittance of red and green 550nm wavelengths of light [32]. For production and marketing purposes, this colour would have minimal impact on most consumer items, which do not require full transparency. If necessary, the yellow tint could be minimised with lower chitosan levels or masked with other pigments. The reduced performance of the control samples at higher temperatures is due to the re-hydrolysis process, breaking down the collagenous fibrils even more. These shortened fibrils then form a matrix around water molecules, increasing the fluid volume; elevated temperatures lead to removal of water content, which causes dramatic shrinkage [5]. However, due to the non-homogeneous composition of CP2-thin film, shrinkage results are subject to variability, as the partially hydrolysed collagen and collagenous matrix shrink at different rates.
Degradation rate experiments show that CP2-thin film undergoes some mass loss in aquatic environments, indicating that despite the insoluble nature of chitosan, the film retains enough water solubility to biodegrade whilst maintaining a higher shelf life and lower susceptibility to moisture in other environments (Fig. 12) [27, 22]. Higher biodegradation rates of CP2-thin film in soil as compared to PLA makes it a potential candidate for home composting (Fig. 11); as demonstrated, CP2-thin film may enhance plant growth due to the microorganisms in soil and on legume root nodules breaking down and taking up the monomers of its structure (Fig. 10) [4, 28]. All plant roots showed healthy growth and penetration into the soil, though some plants had 1-3% reduced growth at higher CP2 quantities of 30% (w/w) in soil. This may be due to oversaturation of CP2-thin film, wherein disproportionate ratios may slow down the biodegradation rate. Additionally, every seed is subject to minor variances in growing conditions, which may account for some variability in plant sizes. Higher concentrations of CP2 take longer to degrade as more CP2 mass is consumed by microorganisms in the container. Further investigation is needed to determine the end-life potential of CP2-thin film to act as a fertiliser and to assess its impact on the environment.
Potential Uses of Cyclo.Plas 2
As a thin film, CP2 shows potential as both non-medical personal protective equipment (PPE) and packaging (Supplementary Data Fig. S1). In producing face shields and barriers to block exhaled aerosols, such as for food service workers and medical staff, water resistance is necessary to maintain its form during usage. As packaging, shelf life and durability are required to properly hold and protect goods. To serve as an environmentally friendly alternative to plastic, CP2 must also be compostable in the environment. Based on these results and prototypes, CP2 has the requisite tensile strength, water resistance, and shelf life to serve its purpose, and demonstrates end-life compostability to minimise its environmental impact.
Conclusions & Implications
Plastic pollution has become an increasingly prevalent global issue due to rising single-use plastic consumption and limited options for the biodegradation of these products. Using biomimicry of the fish scale composition, calcium salts, and collagen, Cyclo.Plas 2 is a novel alternative polymer with dual focus on tackling both plastic degradability and waste accumulation. Cost-effective prototypes were synthesised from fish scale waste components whilst maintaining strength comparable to that of commercial products or non-medical PPE. Cyclo.Plas 2 also serves as a practical disposal solution through valorising 3D-printed PLA waste.
In its composite form, the presence of HAp and SA leads to enhanced degradation in all tested environments, suggesting potential for future use in home composting, as well as indicating a shift away from reliance on scarce industrial facilities to process conventional PLA. As both the thin film and composite, CP2 can function as a “smarter” plastic in replacing grocery bags, food storage, packaging, containers, and novelty items, giving waste a new life and being part of the solution. At the end of its useful life, CP2 could be home composted to promote a circular economy and to tackle the plastic problem. In its thin-film form, CP2 consists of collagenous matrix and chitosan, enhancing tensile strength and water resistance, whilst still maintaining sufficient solubility to degrade safely in the environment with no phytotoxic effects. Additionally, CP2-thin film reduces the current costs of municipal waste management by utilising waste and reducing demand for new material. Further developments include developing CP2-thin film from raw fish scale waste and comparing its physical performance to current samples, expanding degradation testing with a simulated saltwater environment, and directly testing the autoignition point of Cyclo.Plas 2. To synthesise prototypes closer to real-world applications, potential procedures include developing moulds for CP2-composite and converting CP2-composite to a filament for 3D-printing. In developing CP2 to replace single-use plastics, this novel material has the potential of utilising fish scale waste components as adsorbents to remove heavy metals in the aquatic environment or wastewater, as well as promoting home composting and a more circular economy.
Acknowledgements
Special thanks to; Mr. Mario Cuellar from Hitachi, High-Tech America and Dr. Karl Weiss from Arizona State University for the opportunity and their time in teaching and supervising me in using the SEM equipment remotely in this pandemic, Ms. Olson, for putting me in touch with Mr. Cuellar and Mr. Weiss for the SEM access and my family, for always being there to cheer me on and support me the whole way - they are my biggest supporters.
Author's Notes
All figures, or tables, were created by the Author, unless otherwise mentioned in the description provided of said figure.
References
[1] O.R. Peñarubia, “Fish Waste Management: Turning fish waste into healthy feed,” Food and Cultural Organization of the UN, December 2017. [Online]. Available: http://www.fao.org/fi/static-media/MeetingDocuments/WorkshopAMR17/presentations/22.pdf. [Accessed 24 July 2019].
[2] S. C. F. Pang, “Extraction Of Collagen From Fish Wastes, Optimization And Characterization,” Doctoral Dissertation UTAR, 2016. [Online]. Available: https://pdfs.semanticscholar.org/8a01/22af7e6879aaea7d5f2749fb077b46ad214f.pdf. [Accessed 24 July 2019].
[3] M. McGrouther. “Cycloid and Ctenoid Scales,” Australian Museum, February 2019. [Online]. Available: https://australianmuseum.net.au/learn/animals/fishes/cycloid-and-ctenoid-scales/. [Accessed July 5, 2019].
[4] S. Chuaychan. “Production and Characterization of Collagen, Gelatin and Gelatin Hydrolysate Powder from Scales of Spotted Golden Goatfish,” DocPlayer, 2016. [Online]. Available: https://docplayer.net/128290891-Production-and-characterization-of-collagen-gelatin-and-gelatin-hydrolysate-powder-from-scales-of-spotted-golden-goatfish-sira-chuaychan.html. [Accessed 23 June 2019].
[5] H. Lodish et al., Collagen: The Fibrous Proteins of the Matrix, 4th edition. New York: W. H. Freeman; 2000.
[6] MatWeb LLC. “Calcium Hydroxyapatite,” 2019. [Online]. Available: http://www.matweb.com/search/datasheet_print.aspx?matguid=e1654c43ab994d7fab5e0f9aabe4dddc. [Accessed 26 July 2020].
[7] Fluidinova. “Hydroxyapatite: Properties, Uses, and Applications,” 2019. [Online]. Available: https://www.fluidinova.com/hydroxyapatite-properties-uses-and-applications. [Accessed 24 June 2021].
[8] V. S. Kattimani, S. Kondaka, and K. P. Lingamaneni, "Hydroxyapatite–-Past, Present, and Future in Bone Regeneration," Bone and Tissue Regeneration Insights, vol. 7, 2016. Available: https://doi.org/10.4137/BTRI.S36138.
[9] J. Christoffersen, M. R. Christoffersen, and N. Kjaergaard, "The kinetics of dissolution of calcium hydroxyapatite in water at constant pH," Journal of Crystal Growth, vol. 43, no. 4, pp. 501-511, 1978. Available: https://doi.org/10.1016/0022-0248(78)90350-0.
[10] M. J. Larsen, E. I. F. Pearce, and S. J. Jensen, "Notes on the Dissolution of Human Dental Enamel in Dilute Acid Solutions at High Solid/Solution Ratio," Caries Research, vol. 27, no. 2, pp. 87-95, 1993. Available: https://doi.org/10.1159/000261523.
[11] UN Environment, “#BeatPlasticPollution This World Environment Day,” 2019. [Online]. Available: https://www.unenvironment.org/interactive/beat-plastic-pollution/. [Accessed 26 September 2020].
[12] Scientific American, “The Environmental Impact of Corn-Based Plastics,” July 2008. [Online]. Available: https://www.scientificamerican.com/article/environmental-impact-of-corn-based-plastics/. [Accessed 26 September 2020] .
[13] A. Barrett, “Is PLA Recyclable?,” Bioplastics news, April 2020. [Online]. Available: https://bioplasticsnews.com/2020/04/05/is-pla-recyclable/. [Accessed 22 November 2020].
[14] X. Ran, X. Yu, J. He, and Z. Xiao, "Molecular Mechanism of Nano-Hydroxyapatite Surface Changes from Hydrophilic to Hydrophobic," Asian Journal of Chemistry, vol. 26, no. 17, pp. 5355-5359, 2014. Available: https://doi.org/10.14233/ajchem.2014.18110.
[15] J. Russias, E. Saiz, R. K. Nalla, K. Gryn, R. O. Ritchie, and A. P. Tomsia, "Fabrication and mechanical properties of PLA/HA composites: A study of in vitro degradation," Materials Science and Engineering: C, vol. 26, no. 8, pp. 1289-1295, 2006. Available: https://doi.org/10.1016/j.msec.2005.08.004.
[16] Beyond Benign, “Recycling Polylactic Acid (PLA) - Student Guide,” 2020. [Online]. Available: https://www.beyondbenign.org/bbdocs/pdfs/Lactic_Acid_Titration_Extension.pdf. [Accessed 21 October 2020].
[17] Wisconsin Chem Pages, “Solubility and pH,” 2020. [Online]. Available: https://www2.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/acid_base/04salts/salt4.htm. [Accessed 15 November 2020].
[18] Biard & Crockett, “Why Hard Water Doesn’t Get Along with Soap,” 2021. [Online]. Available: https://www.biardandcrockett.com/blog/why-hard-water-doesnt-get-along-with-soap/. [Accessed 20 December 2020].
[19] R. Goldy, “Anions and cations in plants, oh my! But why do we care?,” MSU, December 2013. [Online]. Available: https://www.canr.msu.edu/news/anions_and_cations_in_plants_oh_my_but_why_do_we_care. [Accessed 10 January 2021].
[20] A. Grząbka-Zasadzińska, T. Amietszajew, and S. Borysiak, "Thermal and mechanical properties of chitosan nanocomposites with cellulose modified in ionic liquids," Journal of Thermal Analysis and Calorimetry, vol. 130, no. 1, pp. 143-154, 2017. Available: https://doi.org/10.1007/s10973-017-6295-3.
[21] Encyclopedia Britannica, “Integument,” 2020. [Online]. Available: https://www.britannica.com/science/integument/Arthropods#ref248948. [Accessed 11 December 2020].
[22] E. Leikina, M. V. Mertts, N. Kuznetsova, and S. Leikin, "Type I collagen is thermally unstable at body temperature," Proceedings of the National Academy of Sciences, vol. 99, no. 3, pp. 1314-1318, 2002. Available: https://doi.org/10.1073/pnas.032307099.
[23] MatWeb LLC, “Overview of materials for Low Density Polyethylene (LDPE), Film Grade,” 2019. [Online]. Available: http://www.matweb.com/search/datasheet.aspx?MatGUID=9ff98d958a714b2a8a00990a929d6f14. [Accessed 22 August 2019].
[24] MatWeb LLC, “Overview of materials for Polylactic Acid (PLA) Biopolymer,” 2019. [Online] Available: https://www.matweb.com/search/DataSheet.aspx?MatGUID=ab96a4c0655c4018a8785ac4031b9278. [Accesseed 22 August 2020].
[25] American Society for Testing Materials, “ASTM D882 - 18,” ASTM International, 2021. [Online]. Available: https://www.astm.org/Standards/D882.
[26] Organisation for Economic Cooperation and Development, “OECD Guidelines for the Testing of Chemicals, Section 2,” 2021. [Online]. Available: https://www.oecd-ilibrary.org/environment/test-no-208-terrestrial-plant-test-seedling-emergence-and-seedling-growth-test_9789264070066-en
[27] American Society for Testing Materials, “ASTM D1746 - 15,” ASTM International, 2021. [Online]. Available: https://www.astm.org/Standards/D1746.htm
[28] American Society for Testing Materials, “ASTM D1204 - 14(2020),” ASTM International, 2021. [Online]. Available: https://www.astm.org/Standards/D1204.htm
[30-29] Exploratorium, “Color Table,” 2020. [Online]. https://www.exploratorium.edu/snacks/color-table. [Accessed 11 December 2020].
[30] C. Qin, H. Li, Q. Xiao, Y. Liu, J. Zhu, and Y. Du, "Water-solubility of chitosan and its antimicrobial activity," Carbohydrate Polymers, vol. 63, no. 3, pp. 367-374, 2006. Available: https://doi.org/10.1016/j.carbpol.2005.09.023.
[31] C. Xu and B. Mou, "Chitosan as Soil Amendment Affects Lettuce Growth, Photochemical Efficiency, and Gas Exchange," HortTechnology, vol. 28, no. 4, pp. 476-480, 2018. Available: https://doi.org/10.21273/horttech04032-18.
[32] K. Mikula et al., "3D printing filament as a second life of waste plastics—a review," Environmental Science and Pollution Research, vol. 28, no. 10, pp. 12321-12333, 2020. Available: https://doi.org/10.1007/s11356-020-10657-8.
Supplementary Data
Supplementary data detailing the composition of composites, including ratios of PLA, HAp, and SA used, as well as images of prototypes demonstrating strength properties and potential real-world applications are available in the PDF version of this article.
About This Article
Peer review information: Primary Handling Editor: Lucy Mbochi. Managing Editor: Connie Siu. Youth STEM Matters thanks Lucy Chen, Kerena Norris, Kavina Uthayakumaran, William Mehew, Lily Tierney, Deborah Smith and Adam Khan-Qureshi for their contribution to the peer review of this work.
Received: 5 May 2021
Accepted: 18 September 2021
Published: 20 December 2021